Highlights

What is the mass of a neutrino at rest? A team led by the department of Klaus Blaum, Director at the Max Planck Institute for Nuclear Physics in Heidelberg (MIPK), with the participation of Christoph Düllmann's working group at Johannes Gutenberg University Mainz (JGU), the GSI Helmholtzzentrum für Schwerionenforschung in Darmstadt, and the Helmholtz Institute Mainz (HIM) has now made an important contribution to the “weighing” of neutrinos as part of the international ECHo collaboration. ...

Review in Nature Reviews Physics discusses major challenges in the field of superheavy elements and their nuclei and provides an outlook on future developments ...

Superheavy element 114 (flerovium) is a volatile metal An international research team has succeeded in gaining new insights into the chemical properties of the superheavy element flerovium - element 114 - at the accelerator facilities of the GSI Helmholtz Center for Heavy Ion Research in Darmstadt. The measurements show that flerovium is the most volatile metal in the periodic table. Flerovium is thus the heaviest element in the periodic table that has been chemically studied. ...

Gaining a better understanding of the limiting factors for the existence of stable, superheavy elements is a decade-old quest of chemistry and physics. Superheavy elements, as are called the chemical elements with atomic numbers greater than 103, do not occur in nature and are produced artificially with particle accelerators. ...

In a current paper in the Journal of the American Chemical Society (JACS), we report the first ionization potentials (IP1) of the heavy actinides, fermium (Fm, atomic number Z = 100), mendelevium (Md, Z = 101), nobelium (No, Z = 102), and lawrencium (Lr, Z = 103). ...
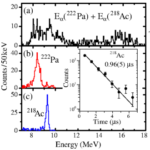
An international team of scientists has succeeded to create and detect extremely short-lived atomic nuclei of the element uranium. ...

Cover story of this week's issue of Nature ...

The synthesis of element 117 at GSI belongs to the Top Ten Physics News Stories in 2014 published by American Physical Society (APS) ...

Life is short for the heaviest elements. They emerge from high-energy nuclear collisions with scant time for detection before they break up into lighter atoms. Even et al. report that even a few seconds is long enough for carbon to bond to the 106th element, seaborgium ...
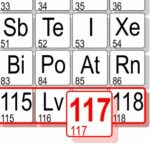
New findings mark important step towards the capability to observe long-lived superheavy nuclei ...
