Highlights

Our recent letter entitled “Probing the Shell Effects on Fission: The New Superheavy Nucleus ²⁵⁷Sg” (P. Mosat et al., Phys. Rev. Lett. 134, 232501 (2025)) has been selected and included in the Physical Review Letters Collection of the Year 2025. ...

The Japanese Kimura Award goes to Dr. Matthias Schädel, the former head of the research department for nuclear chemistry at GSI/FAIR, for his “outstanding and pioneering contributions to the advancement of radiochemistry.” Schädel is the first non-Japanese recipient of this honor. ...

An international research team lead by GSI/FAIR, Johannes Gutenberg University Mainz (JGU) and Helmholtz Institute Mainz (HIM) has succeeded in the production of a new seaborgium isotope. ...
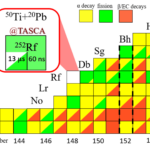
A team of researchers from GSI/FAIR, Johannes Gutenberg University Mainz and the Helmholtz Institute Mainz has succeeded in exploring the limits of the so-called island of stability within the superheavy nuclides more precisely by measuring the superheavy rutherfordium-252 nucleus, which is now the shortest-lived known superheavy nucleus. ...

Experiments at GSI/FAIR succeed in determining properties of moscovium and nihonium. An international team led by scientists of GSI/FAIR in Darmstadt, Johannes Gutenberg University Mainz and the Helmholtz Institute Mainz, succeeded in determining the chemical properties of the artificially produced superheavy elements moscovium and nihonium (elements 115 and 113). ...

What is the mass of a neutrino at rest? A team led by the department of Klaus Blaum, Director at the Max Planck Institute for Nuclear Physics in Heidelberg (MIPK), with the participation of Christoph Düllmann's working group at Johannes Gutenberg University Mainz (JGU), the GSI Helmholtzzentrum für Schwerionenforschung in Darmstadt, and the Helmholtz Institute Mainz (HIM) has now made an important contribution to the “weighing” of neutrinos as part of the international ECHo collaboration. ...

Review in Nature Reviews Physics discusses major challenges in the field of superheavy elements and their nuclei and provides an outlook on future developments ...

Superheavy element 114 (flerovium) is a volatile metal An international research team has succeeded in gaining new insights into the chemical properties of the superheavy element flerovium - element 114 - at the accelerator facilities of the GSI Helmholtz Center for Heavy Ion Research in Darmstadt. The measurements show that flerovium is the most volatile metal in the periodic table. Flerovium is thus the heaviest element in the periodic table that has been chemically studied. ...

Gaining a better understanding of the limiting factors for the existence of stable, superheavy elements is a decade-old quest of chemistry and physics. Superheavy elements, as are called the chemical elements with atomic numbers greater than 103, do not occur in nature and are produced artificially with particle accelerators. ...

In a current paper in the Journal of the American Chemical Society (JACS), we report the first ionization potentials (IP1) of the heavy actinides, fermium (Fm, atomic number Z = 100), mendelevium (Md, Z = 101), nobelium (No, Z = 102), and lawrencium (Lr, Z = 103). ...
